FDA Launches 'TEMPO' Pilot Program to Revitalize Digital Therapeutics Market
New regulatory model aims to overcome limitations of existing FDA approval process
The Light and Shadow of Digital Therapeutics
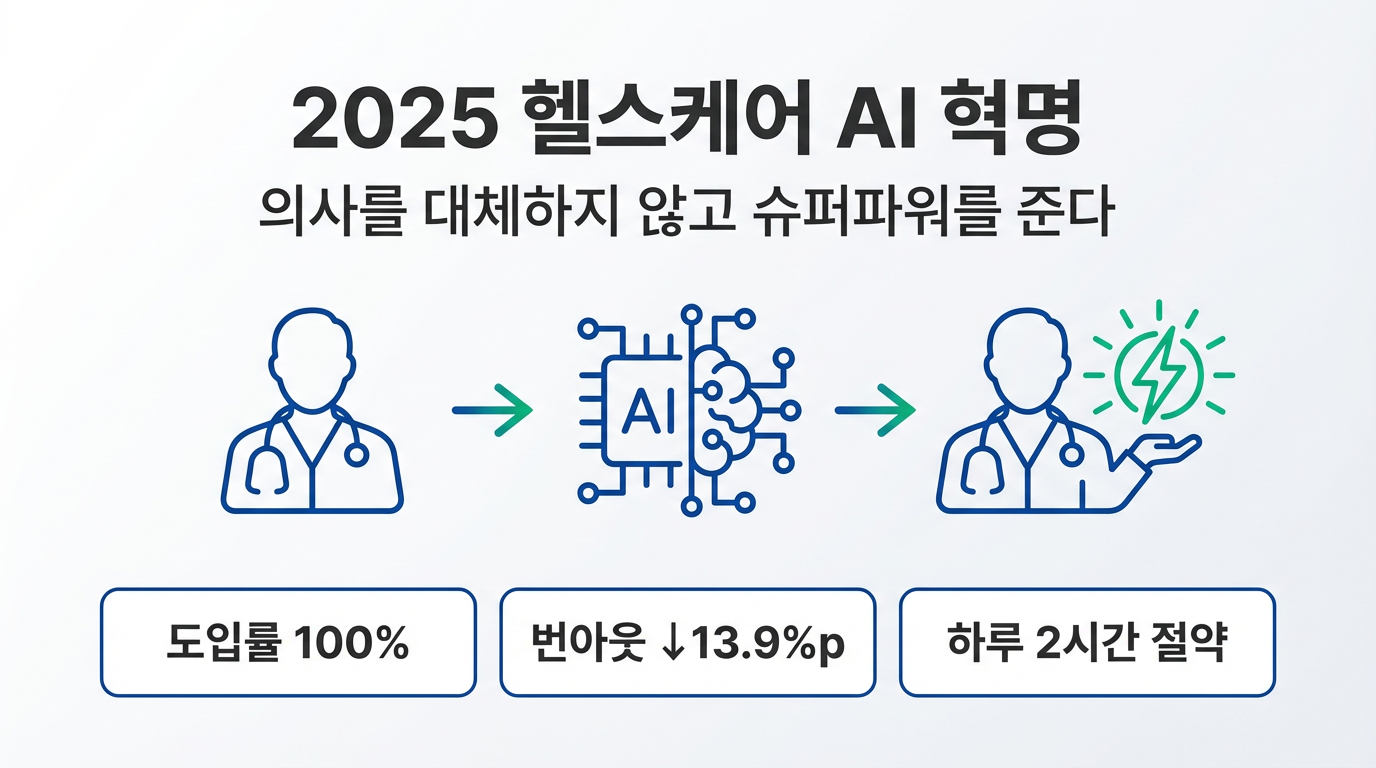
Digital therapeutics are software-based medical devices prescribed to treat specific diseases or disorders, regulated by the FDA. As of December 2024, the FDA has cleared 192 DTx devices, but the industry's overall performance has fallen short of expectations.
A prime example is Pear Therapeutics, which received the first DTx device clearance in 2017. The company priced its apps at over $1,000 but declared bankruptcy in 2023 after failing to establish a sustainable reimbursement strategy. Digital medicine company Akili Interactive also pivoted from a prescription to over-the-counter model following poor sales in 2023, before selling off its assets in 2024 and effectively exiting the market.

Core of the TEMPO Model: Pre-Clearance Prescribing
The most distinctive feature of the TEMPO model is that it allows certain medical professionals to prescribe DTx devices to patients before they receive FDA clearance. Real-world performance data collected through this process will then inform the FDA clearance review.
Vaile Wright, Senior Director of Healthcare Innovation at the American Psychological Association (APA), noted in an interview with Healthcare Brew that "the current FDA clearance pathway is simply not built for software" and "it disincentivizes companies from engaging in that space." She expressed hope that TEMPO could provide a "middle ground" between going straight to market through app stores and going through the FDA clearance process.
Participation in TEMPO is limited to prescribers who are part of the separate CMS Center for Medicare and Medicaid Innovation program known as 'ACCESS (Advancing Chronic Care with Effective, Scalable Solutions).' Since both programs require outcome-based data, combining them could offer companies a two-for-one benefit.
Medicare Coverage Falls Short of Expectations
The DTx industry anticipated 2025 as a breakthrough year. After years of industry advocacy, Medicare began covering digital therapeutics, with seven digital mental health treatment devices initially eligible for reimbursement.
However, more than a year later, expert assessments are divided. According to an analysis released on January 29 by healthcare analytics AI company Komodo Health, 446 patients across 897 visits used the new Medicare billing codes through Q3 2025—a significant increase from 99 patients across 184 visits in Q1 2025.
Evan Woodard, Senior Clinical Product Manager at Komodo Health, offered an optimistic assessment: "Digital mental health therapies are showing a clear inflection point, moving beyond pilots and into mainstream clinical workflows."
Wright, however, evaluated that the results "did not meet their potential." She pointed to CMS's decision not to set a national rate, instead delegating this task to regional Medicare contractors, as problematic. "It's been clear from our conversations with them that they need more direction," she said.
A CMS spokesperson stated that they "lacked sufficient, standardized data" to set a national rate but remained "open to setting one in the future as the evidence base matures."
Implications
The launch of the TEMPO program presents new possibilities for the digital therapeutics industry. Gathering real-world clinical data through pre-clearance prescribing can lower market entry barriers for companies while providing the FDA with more substantive review evidence. However, the industry consensus is that sustainable growth will require parallel improvements to the insurance reimbursement system.
One-line Summary: The FDA has launched the 'TEMPO' pilot program allowing pre-clearance prescribing of digital therapeutics, opening a new pathway for the DTx industry that has struggled with high regulatory barriers and lack of insurance reimbursement.

![[San Lee's Perspective]왜 지구 최극단의 장소들이 에이전틱 AI의 미래를 결정하게 될까 (Part 1)](https://cdn.media.bluedot.so/bluedot.mediontech/2026/01/brfmue_202601231704.webp)