Medtronic Nets FDA Clearance for AI-Powered Robotic Spine System — Stealth AXiS Aims to Cement Leadership in $15 Billion Market
Next-generation platform integrates Mazor robotics, StealthStation navigation, and AiBLE AI ecosystem — as J&J's DePuy Synthes prepares for spin-off and Stryker reshapes its spine portfolio
Medtronic has received U.S. Food and Drug Administration (FDA) clearance for Stealth AXiS, a next-generation spine surgery system that unifies AI-based surgical planning, real-time intraoperative navigation, and robotic assistance in a single integrated platform.
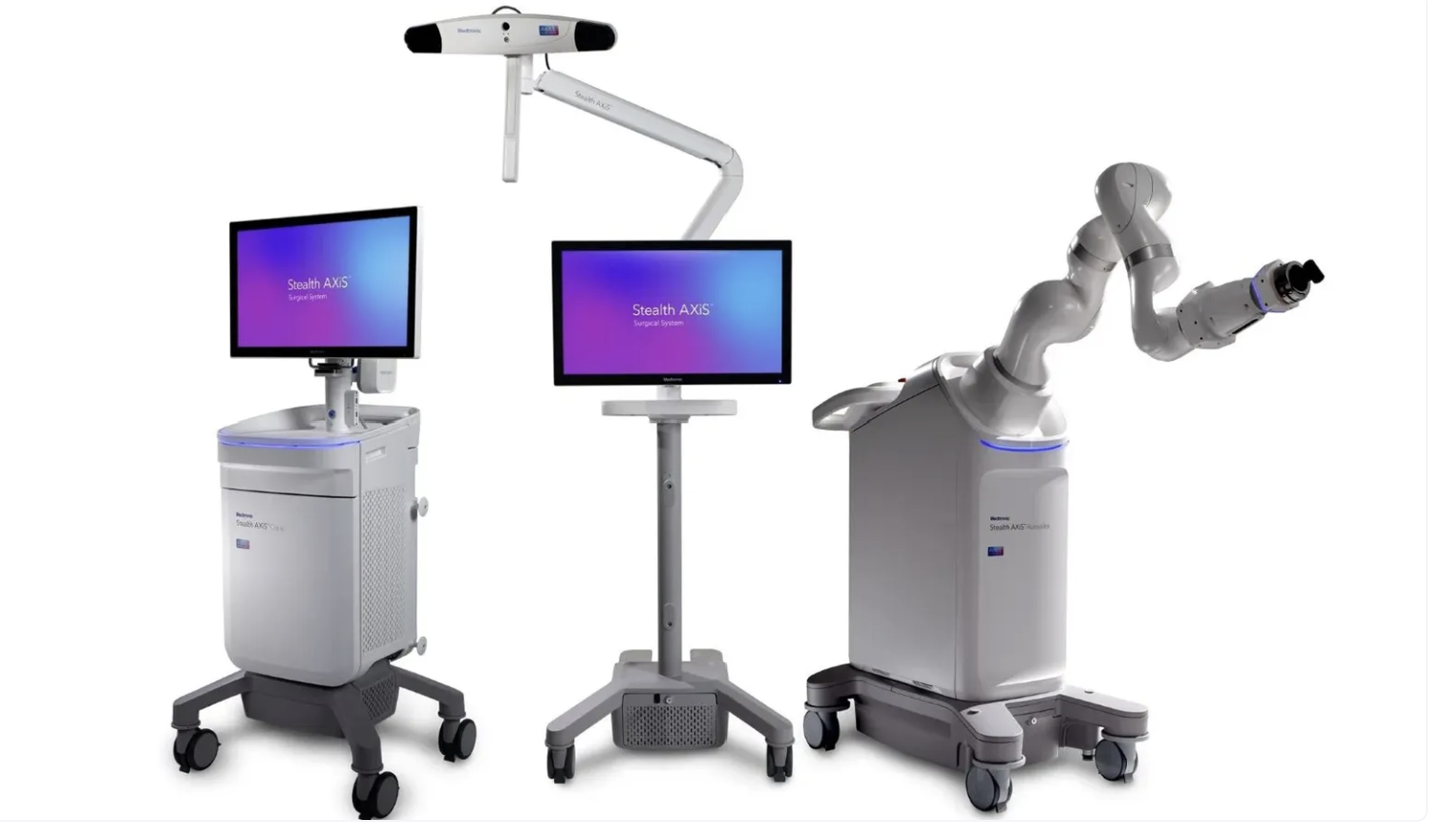
The clearance — arriving eight years after Medtronic's $1.64 billion acquisition of Mazor Robotics in 2018 — marks the company's most advanced AI-robotics convergence in surgical technology, and comes as Medtronic tells investors it is actively gaining share in the $15 billion cranial and spinal technologies market.
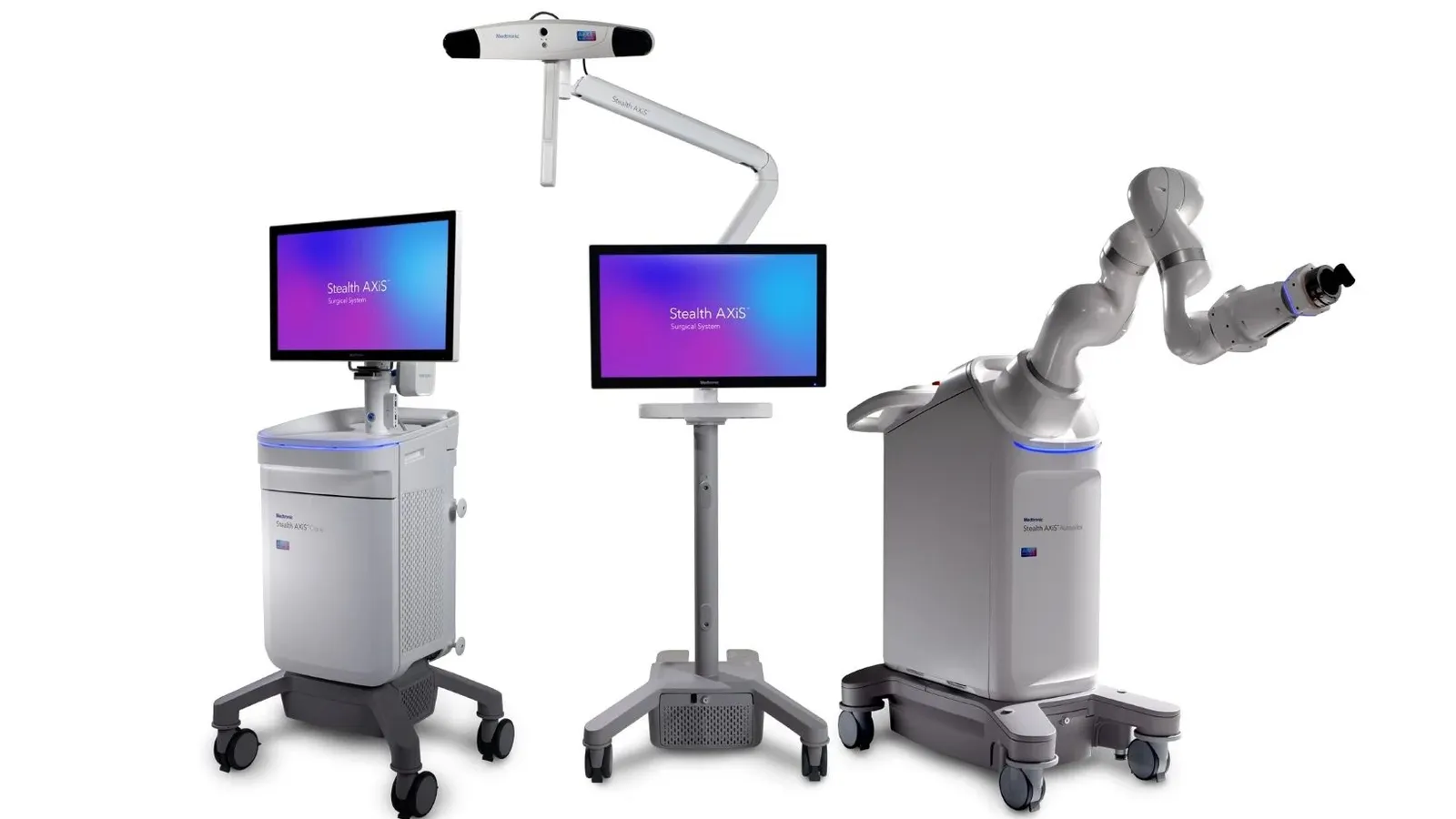
Table 1. Stealth AXiS — Specifications and Clearance Overview
Source: Medtronic official announcement; Healthcare Dive (Feb. 18, 2026)
Real-Time Visualization Without Repeat Imaging — The Core Clinical Advance
The defining clinical capability of Stealth AXiS is the ability for surgeons to visualize anatomic motion, surgical adjustments, and patient alignment in real time during spine procedures — without requiring repeated intraoperative imaging. Conventional spine surgery has relied on periodic fluoroscopic imaging to confirm instrument positioning, exposing patients and OR teams to repeated radiation doses. Medtronic's integration of Mazor's robotic precision with StealthStation's navigation platform directly addresses this longstanding limitation.
The system's modular architecture is designed to accommodate diverse surgeon preferences, and it is deployable across both inpatient hospital operating rooms and ambulatory surgery centers (ASCs) — outpatient facilities where patients are discharged the same day. The ASC segment has grown rapidly as cost pressures and patient preference drive surgical volume out of hospital settings.
Stealth AXiS connects with Medtronic's AiBLE ecosystem — a platform combining AI, data, and services to support personalized pre-operative planning, intraoperative execution, and post-operative outcome analysis across the full patient journey. Medtronic has disclosed that AiBLE's installed base is ten times larger than the nearest competitor's, a data compounding advantage with each incremental procedure. Medtronic plans to pursue additional 510(k) clearances to expand Stealth AXiS into cranial and ENT applications.
Table 2. AiBLE Ecosystem — Component Overview
Source: Medtronic official announcement
"AiBLE ecosystem installed base is ten times larger than the nearest competitor." — Medtronic
$15 Billion Market at a Technology Inflection Point
At the J.P. Morgan Healthcare Conference this year, Medtronic told investors it views the cranial and spinal technologies market as an attractive segment at a 'technology inflection point' — where the shift from conventional implant-based business models to integrated AI, data, and robotics platforms creates durable competitive differentiation. The Siemens Healthineers partnership extends this logic: by integrating AiBLE with the Multitom Rax robotic X-ray system for pre- and post-operative imaging, Medtronic constructs a connected end-to-end surgical workflow that raises switching costs for any hospital deeply embedded in the AiBLE ecosystem.
Competitive Reshaping: J&J Spin-Off and Stryker's Restructuring Create an Opening
Johnson & Johnson announced in October 2025 its intention to spin out DePuy Synthes orthopedics — including its spine robotics and navigation platform — within 18 to 24 months. Corporate separations are inherently disruptive to sales force continuity, customer relationships, and R&D alignment, creating a window for competitors to accelerate account capture. Stryker sold its U.S. spinal implants business to VB Spine (established by Viscogliosi Brothers), retaining its interventional spine, neurotechnology, and enabling technologies divisions and maintaining a strategic partnership with VB Spine. Stryker separately obtained FDA clearance for its OptaBlate lower back pain treatment device in May 2025. With both rivals in structural transition, Medtronic's timing of the Stealth AXiS launch against its 10x installed base advantage appears strategically calculated.
Table 3. $15 Billion Cranial & Spinal Market — Competitive Landscape
Source: Company announcements; Healthcare Dive analysis (Feb. 2026)
Company Profiles
Table 4. Key Company Profiles
Source: Company official filings and announcements
Implications for the Korean Market
South Korea presents a distinctive case study for Stealth AXiS market expansion. The country operates one of the world's highest per-capita volumes of spine surgery — a function of an aging population, high rates of degenerative spinal disease associated with sedentary work culture, and one of Asia's most accessible healthcare systems. The convergence of these factors makes Korea a strategically important early-adoption market for premium spine robotics platforms.
For Medtronic Korea, the clearance accelerates an existing trajectory. The company has been building AiBLE ecosystem presence across major Korean tertiary hospitals, and Stealth AXiS filing with the Ministry of Food and Drug Safety (MFDS) is expected to be a near-term priority. If approved, deployment would initially concentrate in high-volume spine centers — Seoul National University Hospital, Severance Hospital, Samsung Medical Center — before cascading into secondary hospital networks.
The competitive implications for Korea's domestic medtech sector are substantial. Companies such as Curexo (surgical robotics) and Intellijoint (navigation) face a more challenging differentiation task as the global standard for spine surgery shifts toward a 3-in-1 integrated AI, navigation, and robotics model. Korean medtech firms must choose between building independent platforms capable of competing across the full workflow, or pursuing integration strategies with global ecosystems — a decision with long-term strategic consequences.
The J&J DePuy Synthes spin-off adds a supply chain dimension. Korean orthopedic and spine device distributors that carry DePuy Synthes products — and the hospital procurement teams that depend on them — should begin assessing vendor diversification timelines well ahead of the formal separation. Meanwhile, Korea's National Health Insurance Service (NHIS) reimbursement framework for robot-assisted spine surgery will need to evolve to accommodate the expanding clinical footprint of integrated AI-robotic platforms — a policy process that typically lags commercial deployment by two to three years.
Table 5. Implications for the Korean Medical Device Market
Source: Editorial analysis; Medtronic Korea market intelligence
ASC (Ambulatory Surgery Center): An outpatient surgical facility where patients undergo procedures and are discharged the same day. A growing share of U.S. — and increasingly Korean — surgical volume is migrating to ASCs for cost and patient experience reasons.510(k): FDA premarket notification pathway allowing device clearance by demonstrating substantial equivalence to a legally marketed predicate device.MFDS: Ministry of Food and Drug Safety — South Korea's regulatory authority for medical devices.NHIS: National Health Insurance Service — administers Korea's universal health coverage system, including reimbursement decisions for surgical technologies.Source: "Medtronic nets FDA clearance for robotic spine system," Healthcare Dive, Susan Kelly, Feb. 18, 2026


![[STARTUP]REPLA Overcomes Recycling Limits of Mixed Plastic Waste with Microbe-Based Selective Degradation Technology](https://cdn.media.bluedot.so/bluedot.mediontech/2026/02/8egi8z_202602030223.png)
